- Stock: In Stock
- Product code: 00-00012996
- SKU: RS-F2-BMDU-01
Impact-resistant BioMed Durable Resin for medical applications
BioMed Durable Resin is a transparent photopolymer for 3D printing specifically designed for medical applications where impact, shatter, and abrasion resistance is important. The USP Class VI material is ideal for a wide range of medical applications that require prolonged contact with skin (>30 days) and mucous membranes (>30 hours) or short-term contact with tissue, bone, and dentine (<24 hours).
BioMed Durable Resin is manufactured at Formlabs’ FDA-registered, ISO 13485-certified facility and is USP Class VI certified, making it suitable for pharmaceutical and drug delivery applications.
Why choose BioMed Durable Resin?
- Biocompatible. This material is approved for prolonged contact with skin and mucous membranes, as well as for short-term contact with tissue, bone and dentine.
- Certified medical material. Resin is manufactured at Formlabs’ FDA-registered, ISO 13485-certified facility and is also USP Class VI certified. This makes it ideal for use in pharmaceutical and medical applications.
- Shockproof, durable and strong. Parts made from BioMed Durable Resin can withstand impacts of up to 98 J/m, making them resistant to damage.
- Transparent. The resin has excellent transparency, which allows you to create aesthetically pleasing and functional parts.
- Easy to process. The surface of printed parts is smooth and easy to process.
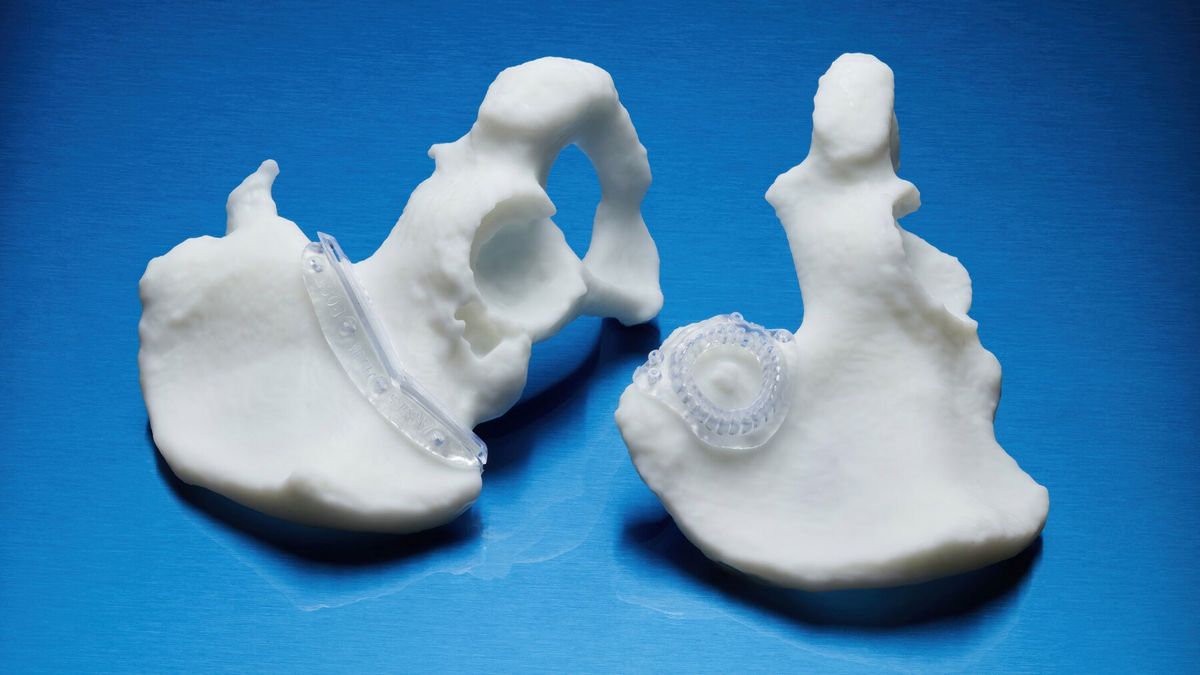
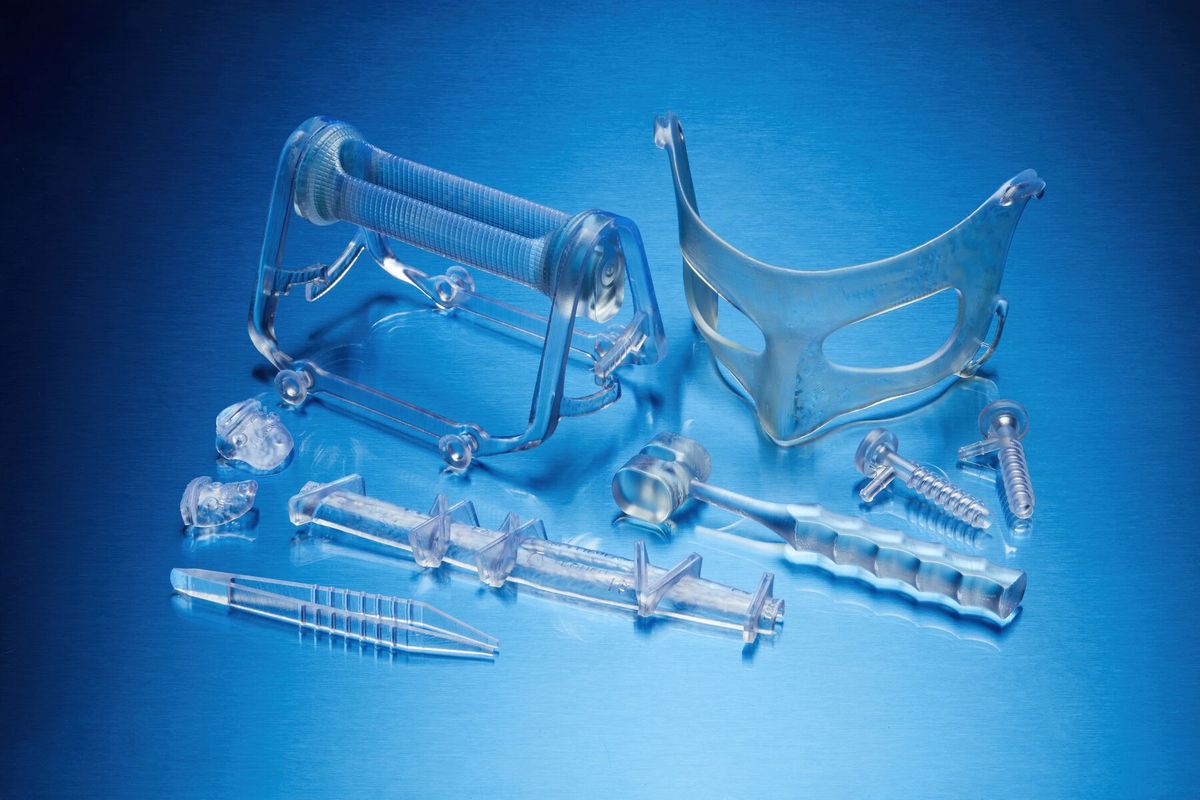
Applications*
- Patient-Specific Instruments (PSI). It can be used to create innovative PSI and medical devices used directly at the point of care.
- Specialty instruments. This resin is suitable for the manufacture of end-use instruments and components that require biocompatibility and impact resistance.
- Surgical instruments. The resin can be used to print surgical instruments, devices, and single-use instruments.
*Unlike Formlabs Dental Resins, BioMed resins do not have a specific intended use. Material properties may vary depending on part design, manufacturing methods, and other techniques. It is the responsibility of the manufacturer, its respective customers and end users to determine the biocompatibility and performance of all printed parts for their respective application and use. To instill confidence in potential users, Formlabs has tested biocompatibility for standard use cases and manufactures BioMed Resins in an ISO 13485 certified facility. We can offer representative examples of what customers have done with our products and the accompanying documentation, which we make freely available.
Specifications
| Printing Technology | SLA |
| Polymerization | Needed |
| Biocompatibility | Yes |
| Межа міцності на розрив | 29,1 MPa |
| Модуль міцності на розтяг | 994 MPa |
| Подовження при розриві | 33% |
| Сила гнучкості | 21 MPa |
| Модуль пружності при вигині | 643 MPa |
| Твердість Шор D | 75D |
| Ізод з насічками | 98 J/m |
| Температура теплового відхилення при 0,45 МПа | 46 ºC |
| Layer Height (micron) | Form 3B, Form 3B+, Form 3BL – 100 |
| Printer Compatibility | Form 3B, Form 3B+, Form 3BL |
| Resin Tank Compatibility | Form 3 RT V1/V2/V2.1, Form 3L RT V1/V2 |
| Build Platform Compatibility | Form 3 BP, Form 3 BP 2, Form 3 Stainless Steel BP, Form 3L BP |
| Volume (L) | 1 |
| Manufacturer Country | USA |
If you have any questions, please contact us.
Downloads
Technical Data Sheet, PDF (EN)