



- Stock: In Stock
- Product code: 00-00000267
- Shipping Weight: 1.50kg
- SKU: RS-F2-BMWH-01
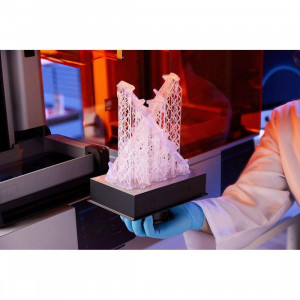
Medical polymer Formlabs BioMed White Resin
Medical polymer Formlabs BioMed White Resin is designed to increase the medical care quality, making modern tools available to every medical institution.
Formlabs is constantly expanding its range of materials for its 3D printers. The line of Formlabs biomedical resins is designed for a wide range of applications where performance and biocompatibility are critical. BioMed Resin materials are developed and manufactured at an ISO 13485 certified plant and are compatible with conventional disinfection and sterilization methods.
Material for 3D printing in medicine
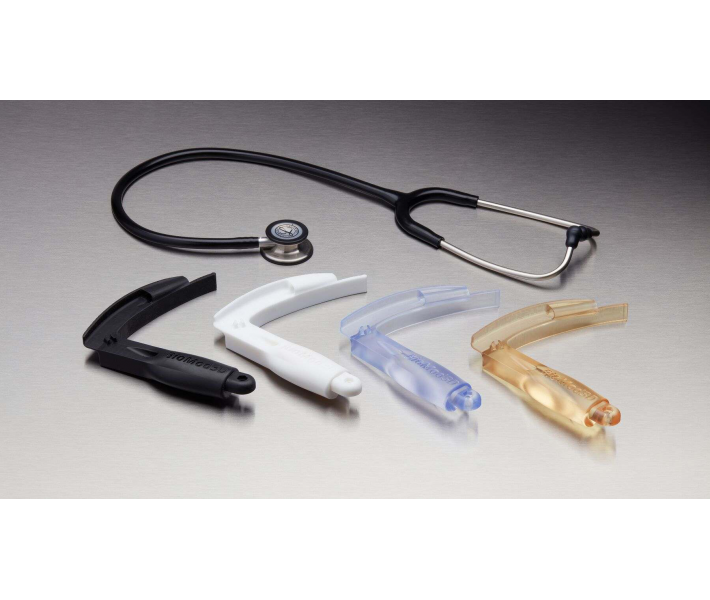
BioMed White Resin is a solid, opaque biocompatible photopolymer designed for cases where prolonged skin contact or short-term contact with the patient’s mucosa is required. This unique medical polymer has been tested for pyrogenicity and acute systemic toxicity in accordance with US Pharmacopoeia standards and can be used in cases where short-term contact with tissues, bones and dentine is expected.
Models printed with BioMed White Resin can be treated with conventional solvent disinfection and sterilization methods. BioMed White Resin is manufactured at an ISO 13485 certified and USP Class VI certified facility, making it suitable for use in pharmaceuticals and drug delivery.
- Printing resolution: 100 microns, 50 microns.
- Final polymerization is required.
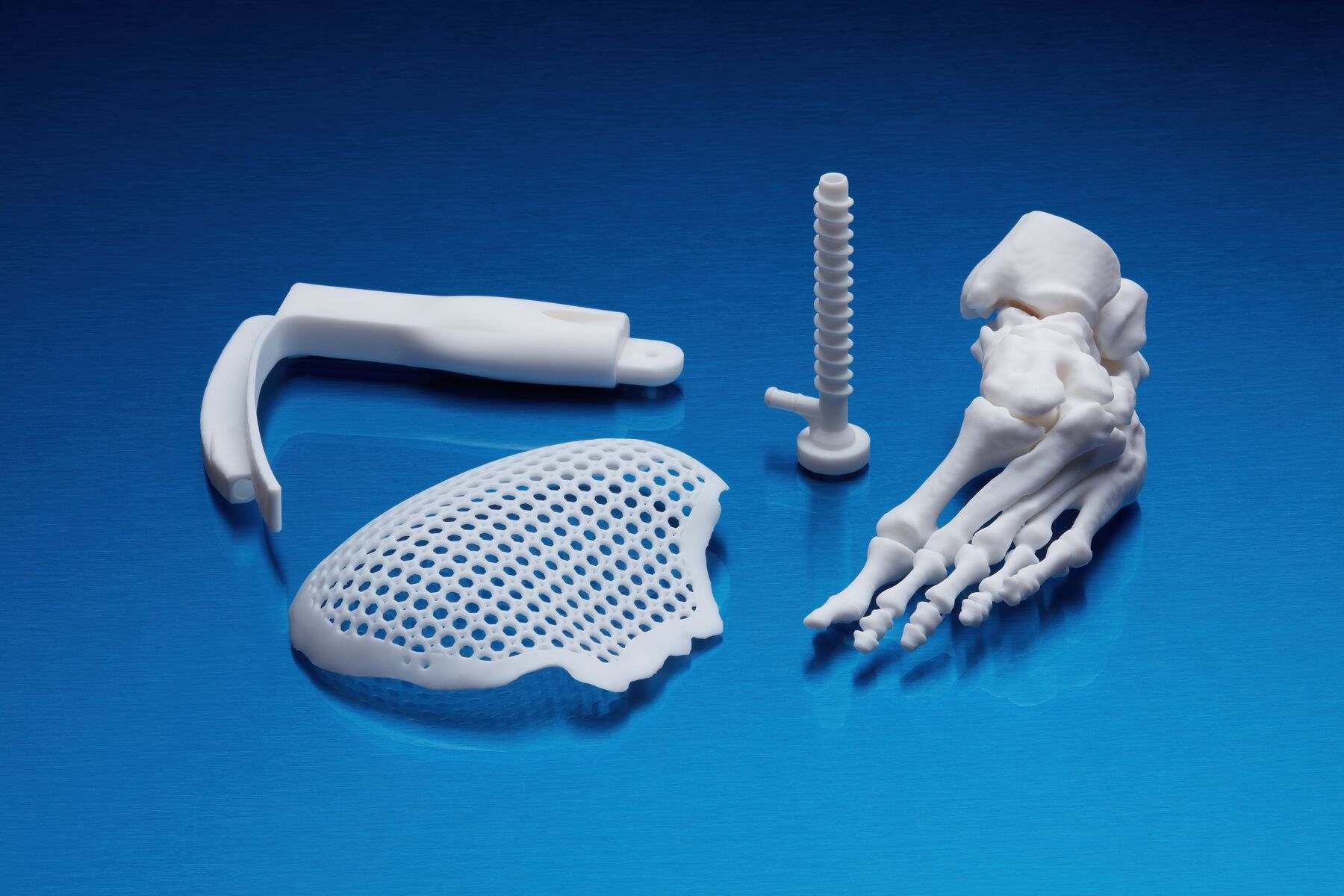
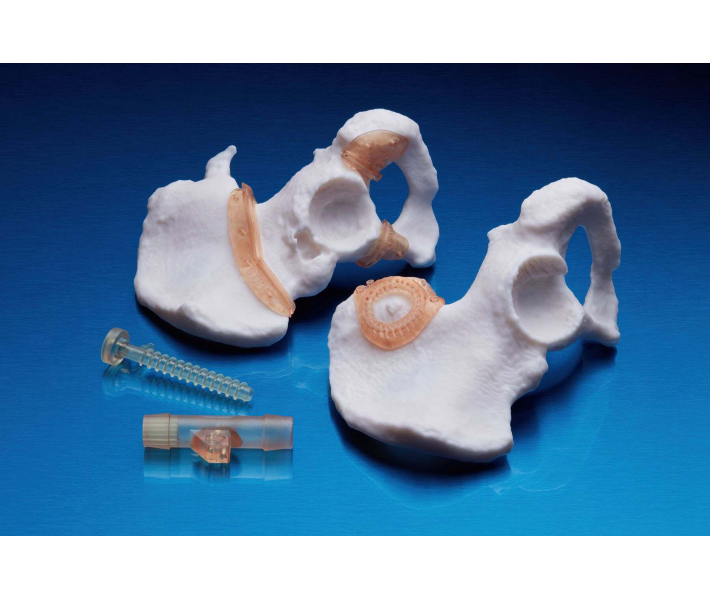
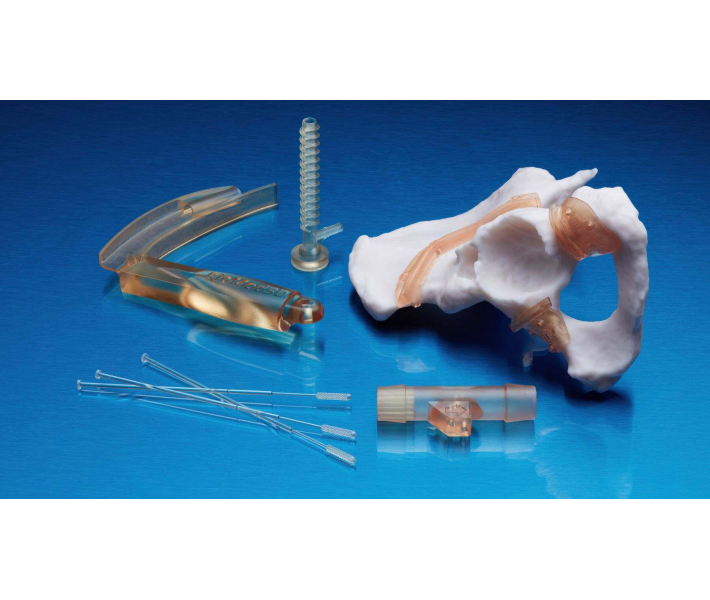
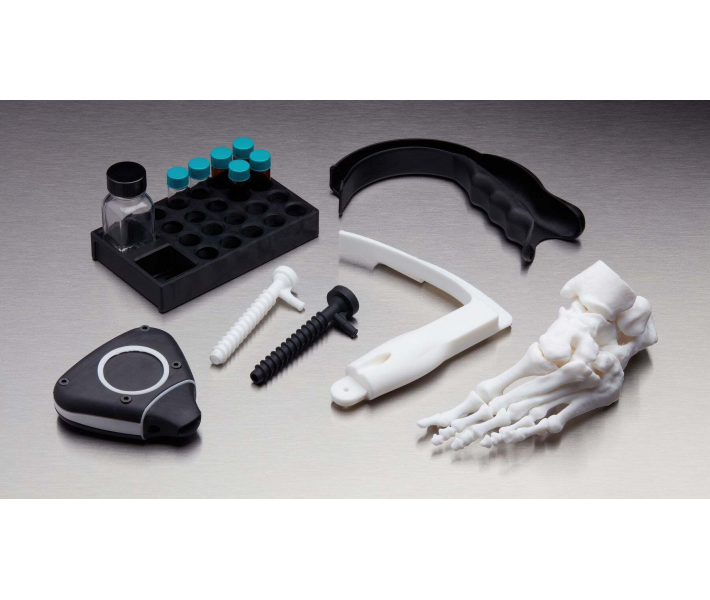
Application of BioMed White Resin
This material will make available of fast customized production:
- End-use medical devices and their components.
- Individual models and molds of implants to determine the size of implants.
- Guides for cutting and drilling.
- Surgical guides and templates.
- Biocompatible molds, clamps, jigs and devices.
- Anatomical models that can be used in the operating room.
BioMed White Resin properties
| Post-Cured1,2 | Method | |
| Tensile Properties | ||
| Ultimate Tensile Strength | 45,78 MPa | ASTM D 638-10 (Type IV) |
| Young’s Modulus | 2020,16 MPa | ASTM D 638-10 (Type IV) |
| Elongation | 10% | ASTM D 638-10 (Type IV) |
| Flexural Properties | ||
| Flexural Stress at 5% Strain | 74,46 MPa | ASTM D 790-15 (Method B) |
| Flexural Modulus | 2020,16 MPa | ASTM D 790-15 (Method B) |
| Hardness Properties | ||
| Hardness Shore D | 80D | ASTM D 2240-15 (Type D) |
| Impact Properties | ||
| Notched IZOD | 15,11 J/m | ASTM D 256-10 (Method A) |
| Unnotched IZOD | 269,03 J/m | ASTM D 4812-11 |
| Thermal Properties | ||
| Heat Deflection Temp. @ 1.8 MPa | 52,4 °C | ASTM D 648-18 (Method B) |
| Heat Deflection Temp. @ 0.45 MPa | 67,0 °C | ASTM D 648-18 (Method B) |
| Coefficient of Thermal Expansion | 90,1 mkm/m/°C | ASTM E 831-13 |
| Other Properties | ||
| Water Absorption | 0,40 wt% | ASTM D570-98 |
| Sterilization Compatibility | |
| E-beam | 35 kGy E-beam radiation |
| Ethylene Oxide | 100% Ethylene oxide at 55 °C for 180 minutes |
| Gamma | 29.4 – 31.2 kGy gamma radiation |
| Steam Sterilization | Autoclave at 134°C for 20 minutes Autoclave at 121°C for 30 minutes |
| Disinfection Compatibility | |
| Chemical Disinfection | 70% Isopropyl Alcohol for 5 minutes |
Biocompatible medical material
Samples printed with BioMed White Resin were evaluated according to the following biocompatibility standards:
| ISO Standard | Description3 |
| ISO 10993-5:2009 | Not cytotoxic |
| ISO 10993-10:2010/(R)2014 | Not an irritant |
| ISO 10993-10:2010/(R)2014 | Not a sensitizer |
| ISO 10993-11: 2017 | No evidence of acute systemic toxicity |
| ISO 10993-11: 2017/USP, General Chapter <151>, Pyrogen Test | Non-pyrogenic |
The product was developed and is in compliance with the following ISO Standards:
| ISO Standard | Description |
| EN ISO 13485:2016 | Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes |
| EN ISO 14971:2012 | Medical Devices – Application of Risk Management to Medical Devices |
Compatibility of BioMed White Resin with solvents
Percent weight gain over 24 hours for a printed and post-cured 1 x 1 x 1 cm cube immersed in respective solvent:
| Solvent | 24 hr weight gain, % | Solvent | 24 hr weight gain, % |
| Acetic Acid, 5 % | 0,4 | Mineral oil, heavy | < 0,1 |
| Acetone | 2,9 | Mineral oil, light | < 0,1 |
| Bleach ~5% NaOCl | 0,3 | Salt Water (3.5% NaCl) | 0,4 |
| Butyl Acetate | 0,4 | Skydrol 5 | 0,5 |
| Diesel Fuel | < 0,1 | Sodium hydroxide solution (0.025% pH = 10) |
0,3 |
| Diethyl glycol monomethyl ether | 1,0 | Strong Acid (HCl Conc) | 0,2 |
| Hydraulic Oil | < 0,1 | TPM | 0,6 |
| Hydrogen peroxide (3%) | 0,3 | Water | 0,3 |
| Isooctane | < 0,1 | Xylene | 0,3 |
| Isopropyl Alcohol | 0,2 |
1 Material properties may vary based on part geometry, print orientation, print settings, temperature, and disinfection or sterilization methods used.
2 Data were measured on post-cured samples printed on a Form 3B with 100 µm BioMed White Resin settings, washed in a Form Wash for 5 minutes in 99% Isopropyl Alcohol, and post-cured at 60 °C, 60 minutes in a Form Cure.
3 BioMed White Resin was tested at NAMSA World Headquarters, OH, USA.