- Stock: In Stock
- Product code: 00-08800070
- Shipping Weight: 1.60kg
- SKU: RS-C2-BMFL-01
Flexible medical BioMed Flex 80A Resin for Form 4B
BioMed Flex 80A flexible medical Resin is a hard, flexible and transparent material for biocompatible applications that require strength and prolonged skin contact (>30 days) or short-term mucosal contact (<24 hours).
BioMed Flex 80A Resin is manufactured in Formlabs’ FDA-registered, ISO 13485-certified facility.
Why choose BioMed Flex 80A Resin?
Achieve new design possibilities and efficiencies using 3D printing and BioMed Flex 80A Resin, from the production of flexible biocompatible medical devices to anatomical models that require biocompatibility.
- Flexible. Produce flexible medical devices or solid tissue models that require short-term mucosal contact, which can optimize the application of mass personalization compared to using bulky molds.
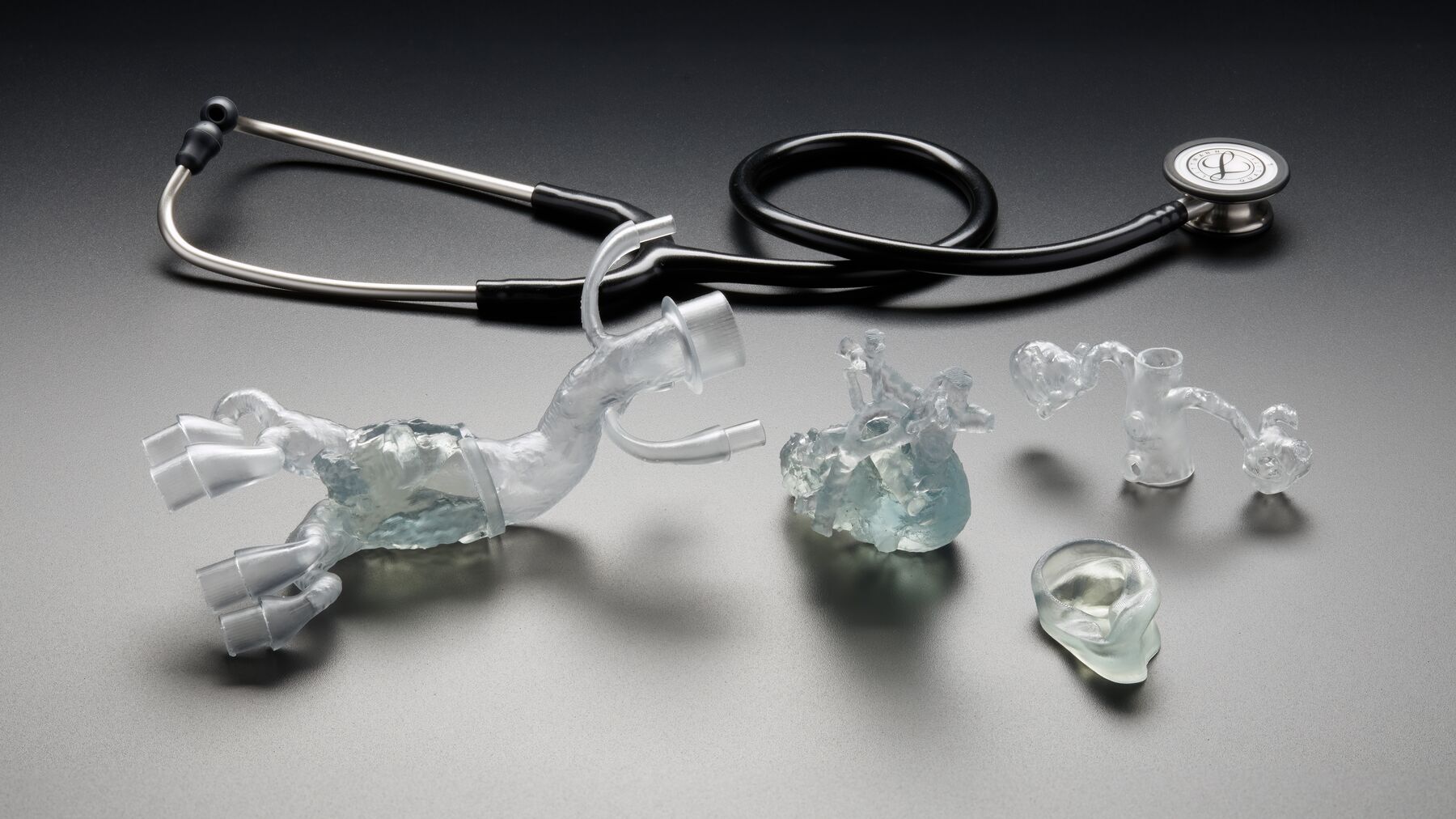
- Transparent. Produce complex, transparent solid tissue models and deliver them directly to the operating room (OR) as a reference for complex medical procedures (e.g., sizing models, cartilage models, or diseased organs).
- Medical material, biocompatible parts. Use material manufactured under our quality management system in strict compliance with ISO 13485. Be assured that this material is suitable for biocompatible applications with prolonged dermal (>30 days), short-term mucosal contact (<24 hours), and USP Class VI certification.
BioMed Flex 80A applications*
Direct 3D printing of flexible parts that require biocompatibility with BioMed Flex 80A Resin. Reduce workflow time by eliminating molding to directly produce flexible, customized medical devices or solid medical tissue models that surgeons can refer to in the operating room.
- Flexible biocompatible medical devices and components.
- Medical devices that require short-term contact with the mucous membrane.
- Solid tissue models to assist during surgery.
*Unlike Formlabs dental resins, BioMed Resins do not have a specific intended use. Material properties may vary depending on part design, manufacturing methods, and other techniques. It is the responsibility of the manufacturer, its respective customers and end users to determine the biocompatibility and performance of all printed parts for their respective application and use. To instill confidence in potential users, Formlabs has tested biocompatibility for standard use cases and manufactures BioMed Resins in an ISO 13485 certified facility. We can offer representative examples of what customers have done with our products and the accompanying documentation, which we make freely available.
Specifications
| Printing technology | SLA |
| Polymerization | Required |
| Biocompatibility | Yes |
| Tensile strength | 7.2 MPa |
| Tensile strength at 50% elongation | 2.6 MPa |
| Tensile strength at break 100% | 4.5 MPa |
| Elongation | 135% |
| Tensile strength | 22 kN/m |
| Hardness Shore D | 80A |
| Layer height (microns) | Form 4B – 100 |
| Printer Compatibility | Form 4B |
| Resin Tank Compatibility | Form 4 Resin Tank |
| Build Platform Compatibility | Form 4 Build Platform, Form 4 Build Platform Flex |
| Volume (L) | 1 |
| Manufacturer Country | USA |
If you have any questions, please contact us.
Downloads
Technical Data Sheet, PDF (EN)