







- Stock: In Stock
- Product code: 00-00013519
- Shipping Weight: 1.50kg
- SKU: RS-C2-BMAM-01
Accelerate medical innovation with Formlabs BioMed Amber Resin Form 4, 1l. This rigid, strong, and translucent material is specifically engineered for a range of biocompatible applications. Manufactured in an FDA-registered, ISO 13485 certified facility, BioMed Amber Resin provides the reliability and performance required for medical device manufacturing, patient-specific surgical tools, and advanced research models. Produce high-definition, sterilizable parts with confidence, right from your desktop.
A Certified Material for Medical Innovation
Manufactured for Quality
Leverage a true medical-grade material for your most critical applications. BioMed Amber Resin is produced with strict adherence to ISO 13485 within Formlabs' Quality Management Systems, ensuring consistency, traceability, and reliability for every part you print.
Validated for Biocompatibility
This resin is suitable for applications requiring long-term skin contact (over 30 days) and short-term mucosal membrane, bone, tissue, and dentin contact (up to 24 hours). Parts can be sterilized using common solvent disinfection and sterilization methods, making them safe for clinical and surgical environments.
High-Performance and Detail
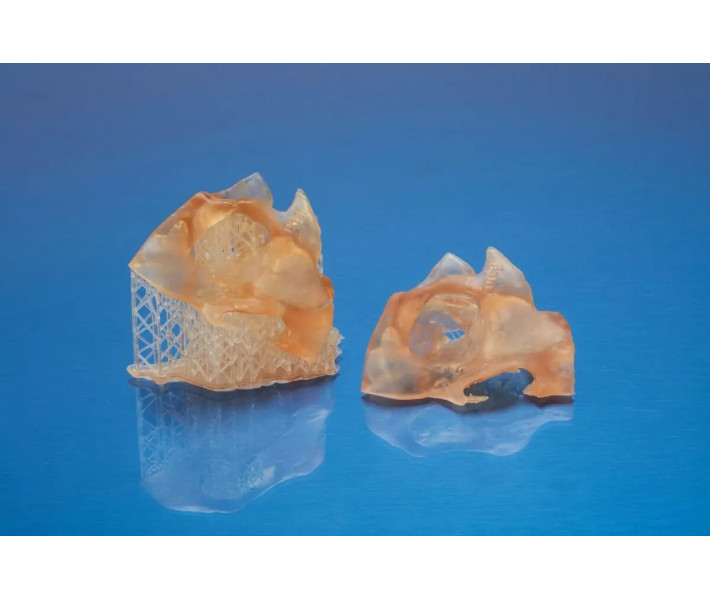
With its high strength and stiffness, BioMed Amber Resin is perfect for producing robust end-use devices and tools that can withstand functional use. The material's semi-transparent nature and excellent definition allow for the creation of parts with highly complex geometries and internal features.
Versatile Medical and Healthcare Applications
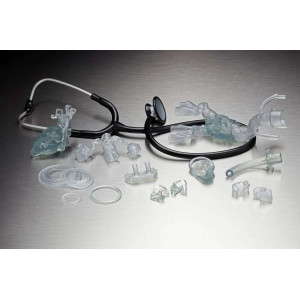
Use BioMed Amber Resin to produce rigid, biocompatible parts for a wide range of point-of-care and medical manufacturing scenarios. It is an excellent choice for:
- End-use medical devices requiring rigidity and biocompatibility.
- Patient-specific implant sizing models for pre-surgical planning.
- Precise surgical cutting and drilling guides.
- Components for medical tools and specimen collection kits.
*It is the responsibility of the device manufacturer to validate the performance and biocompatibility of all printed parts for their specific application and use. Formlabs provides biocompatibility and sterilization testing data for common use cases to support this process.
Material Properties
- Ultimate Tensile Strength: 73 MPa
- Young’s Modulus: 2900 MPa
- Elongation: 12%
- Flexural Strength: 103 MPa
- Flexural Modulus: 2500 MPa
- Hardness Shore D: 67 D
- Notched Izod: 28 J/m
- Heat Deflection Temp @ 0.45 MPa: 78 ºC
Critical Post-Processing for Biocompatibility
To ensure parts meet certified safety standards, proper post-processing is mandatory. Both washing away excess resin and post-curing the part are essential steps to achieve the final biocompatible properties. Please refer to the official manufacturing guide for detailed instructions.
Your Official Formlabs Partner
As an official representative of Formlabs, 3DDevice guarantees the best price, an official warranty, and professional service support. Our experts, with experience since 2012, are here to provide advice and assist you in making the right choice. Rely on us for qualified support before, during, and after your purchase to ensure you achieve the best possible results with your 3D printing projects.