- Stock: In Stock
- Product code: 00-08800069
- SKU: RS-C2-BMDU-11
Impact-resistant medical resin BioMed Durable for Form 4B
Impact-resistant medical resin BioMed Durable is a clear 3D printing material for biocompatible applications that require resistance to impact, shattering, and abrasion.
This USP Class VI material can be used for prolonged contact with skin (>30 days) and mucous membranes (>30 hours) or short-term contact with tissue, bone, and dentin (<24 hours). BioMed Durable Resin is manufactured at Formlabs’ FDA-registered, ISO 13485-certified facility and is USP Class VI certified, making it suitable for pharmaceutical and drug delivery applications.
Why choose BioMed Durable Resin?
Spur medical innovation with 3D printing and BioMed Durable Resin for end-device manufacturing where performance, impact resistance, and biocompatibility are critical.
- Resistant to impact, shattering and abrasion. Manufacture medical-grade parts that are impact resistant with an isoidal impact rating of 98 J/m.
- Certified medical grade material. Use material manufactured under our quality management system in strict compliance with ISO 13485.
- Biocompatible parts. Formlabs’ standard use case testing is designed to give you confidence that this material is suitable for biocompatible applications.
- Excellent transparency and surface finish. Parts made with this material look like end-use parts and have a very smooth surface.
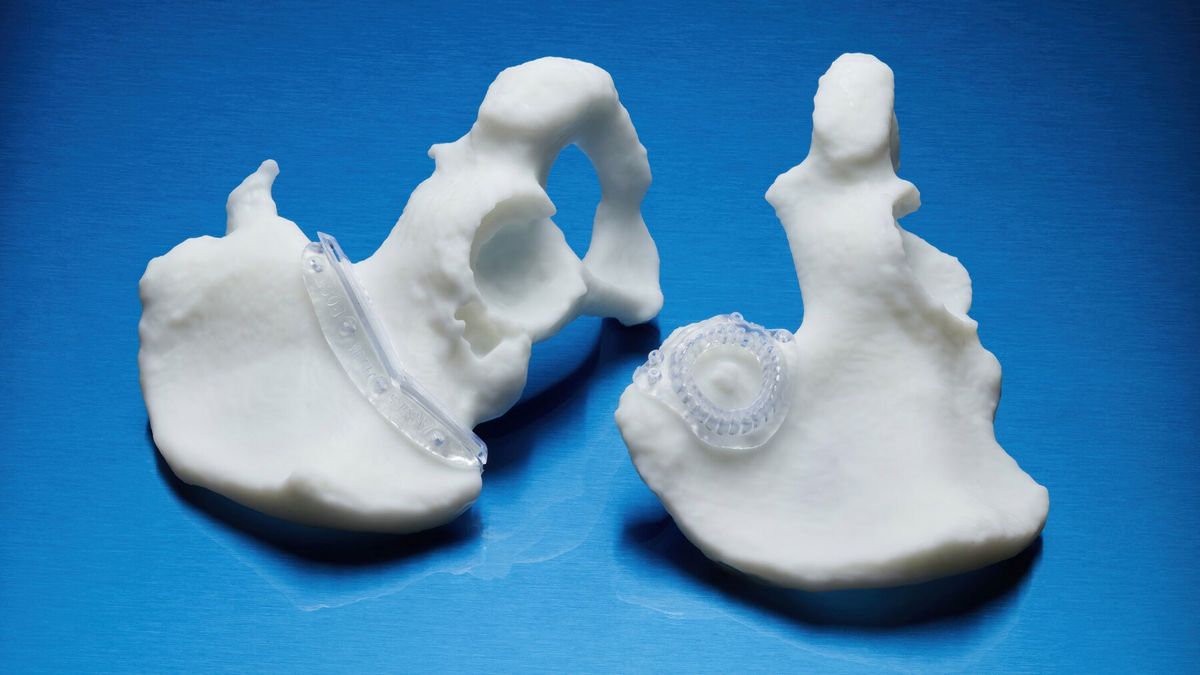
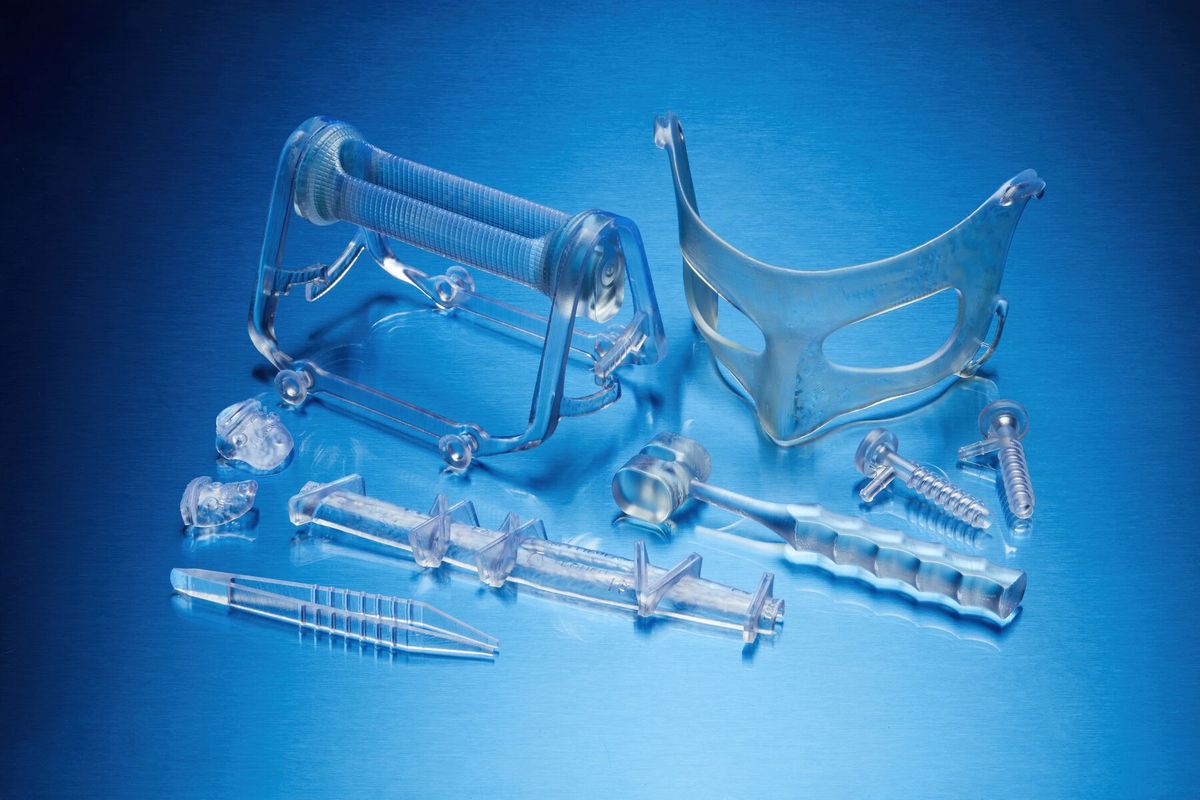
What is printed with BioMed Durable Resin*
Implement patient advanced instruments (PSI) and procedure-specific medical devices at the point of care. Improve patient outcomes, reduce complications and shorten operating times with medical devices printed on BioMed’s durable resin.
- Special tools for the patient.
- End-use devices and components that require biocompatibility and impact resistance.
- Surgical instruments, devices and disposable instruments.
*Unlike Formlabs Dental Resins, BioMed resins do not have an intended use. Material properties may vary depending on part design, manufacturing methods, and other methods. The manufacturer, its respective customers and end users are responsible for determining the biocompatibility and efficacy of all printed parts for their respective application and use. To instill confidence in potential users, Formlabs has tested biocompatibility for standard use cases and manufactures BioMed Resins in an ISO 13485 certified facility. We can offer representative examples of what customers have done with our products and accompanying documentation, which we provide in free access.
Specifications
| Printing technology | SLA |
| Polymerization | Required |
| Biocompatibility | Yes |
| Tensile strength | 29.1 MPa |
| Tensile strength modulus | 994 MPa |
| Elongation at break | 33% |
| Strength of flexibility | 21 MPa |
| Modulus of elasticity in bending | 643 MPa |
| Hardness Shore D | 75D |
| Isod with notches | 98 J/m |
| Thermal deflection temperature at 0.45 MPa | 46 ºC |
| Layer height (microns) | Form 4B – 100 |
| Printer Compatibility | Form 4B |
| Resin Tank Compatibility | Form 4 Resin Tank |
| Build Platform Compatibility | Form 4 Build Platform, Form 4 Build Platform Flex |
| Volume (L) | 1 |
| Manufacturer Country | USA |
If you have any questions, please contact us.